Anticoagulants are among the most powerful tools in modern medicine. They prevent strokes, pulmonary embolism, and other thromboembolic events. But mismanagement can lead to serious bleeding, hospitalizations, and death. According to research, anticoagulants carry an elevated risk of major bleeding, and many patients on warfarin spend a large portion of time outside their therapeutic range. Patients on direct oral anticoagulants (DOACs) face risks from incorrect dosing, renal impairment, and drug interactions.
Traditional quality improvement programs, such as the Michigan Anticoagulation Quality Improvement Initiative (MAQI²), have shown that tracking both clinical outcomes (bleeding, stroke, thromboembolism, hospitalizations, death) and process metrics (time in therapeutic range, delays in responding to out-of-range INR values, appropriate dosing) can reduce adverse events. The challenge is that many institutions struggle with real-time visibility in their EHR or EMR, due to limited, hard to customize functionality of legacy tools. So, even among top performers in the other domains of quality programs, delays in extracting and cleaning data for AC quality management means that interventions happen weeks or months after the risk emerged.
At Adentris, we believe that combining the best of what research recommends with real-time QA of EHR and EMR data can help hospitals and clinics achieve measurable improvements in anticoagulation care, both in outcomes and in financial return. We are inviting providers to participate in a free pilot of our anticoagulation quality improvement initiative.
Adentris in Action: Real-World Examples from the EMR
Real-time QA is only valuable if it surfaces actionable events that clinicians might otherwise miss. Below are examples of how Adentris translates raw EMR data into alerts and integrity checks that prevent harm.
- Excess bleeding risk flag. Example: Patient on Warfarin, Aspirin, and Ibuprofen with renal impairment (GFR 28). Age 82. Alert: “High bleeding risk: concurrent NSAID plus aspirin plus warfarin in patient with renal impairment. Recommend immediate medication reconciliation.” Impact: addresses polypharmacy and comorbidities that increase risk.
- AC drug–drug interaction. Example: Active medication list shows Warfarin 5 mg daily, plus new prescription for Trimethoprim-sulfamethoxazole (Bactrim DS) twice daily. Lab data show stable INR 2.5 for months. Alert: “Bactrim prescribed to patient on warfarin — high risk for INR elevation and bleeding. Recommend alternative antibiotic or close INR monitoring within 48 hours.” Impact: prevents adverse events caused by overlooked interactions.
- Surgery without anticoagulant withdrawal. Example: Encounter note shows patient scheduled for elective knee replacement on October 15. Medication list includes Apixaban 5 mg twice daily active through October 14. No documentation of anticoagulant hold, bridging, or perioperative plan. Alert: “Patient scheduled for surgery in 48 hours with active apixaban prescription and no perioperative anticoagulation plan documented.” Impact: prevents catastrophic bleeding during surgery.
- Out-of-range INR not addressed. Example: Lab result shows INR = 4.7 at 8:35 AM. No clinician note, call, or dose adjustment documented for more than 48 hours. Adentris alert: “Critical INR (4.7) recorded 48 hours ago without provider intervention. Patient at high bleeding risk — escalation required.” Impact: prevents delays that can lead to intracranial hemorrhage, gastrointestinal bleeds, or readmissions.
- High INR variability. Example: Patient INR values over six weeks: 1.8 → 3.9 → 2.0 → 4.2 → 1.7. Wide swings greater than 1.5 units between consecutive draws. Alert: “Patient shows unstable INR control. Review adherence, drug interactions, diet, or consider DOAC.” Impact: identifies patients at highest risk for clotting or bleeding.
- Missed lab draws. Example: Order: INR every 2 weeks. Last documented INR July 3. Current date August 10, no lab result present. Alert: “Patient overdue for INR by more than 3 weeks. Contact patient to schedule lab.” Impact: ensures consistent monitoring for unstable or high-risk patients.
- Data integrity checks. Example: missing labs — INR result ordered but no value posted; wrong dose entry — EMR shows “Warfarin 50 mg daily,” an extra zero entered; missing renal function — patient prescribed dabigatran with no creatinine or GFR recorded. Adentris immediately flags these errors.
Each of these examples represents real clinical risk and financial exposure: hospitalizations for bleeding, surgical complications, malpractice claims, readmission penalties, or missed quality incentives. By embedding automated detection into the EMR, Adentris ensures that none of these events go unnoticed.
What to Measure
Improving quality requires focusing on specific, measurable outcomes. Literature suggests tracking both clinical outcomes and process measures:
- Patient-centered outcomes: bleeding events, strokes, thromboembolic events, hospitalizations, mortality
- Intermediate outcomes: time in therapeutic range, INR variability, percentage of in-range and out-of-range lab values, adherence to monitoring schedules, missed labs
- Process measures: time from initiation of warfarin to first therapeutic INR, delay between out-of-range lab and patient contact, correct DOAC dosing for renal function and indication, appropriate drug choice, documentation of patient education
- Population measures: percentage of patients anticoagulated only when indicated, appropriate duration of therapy, correct INR targets, avoidance of unnecessary concurrent medications that elevate bleeding risk
Improving these measures has been shown to reduce serious adverse events and hospital costs while also raising compliance with payer and regulator expectations.
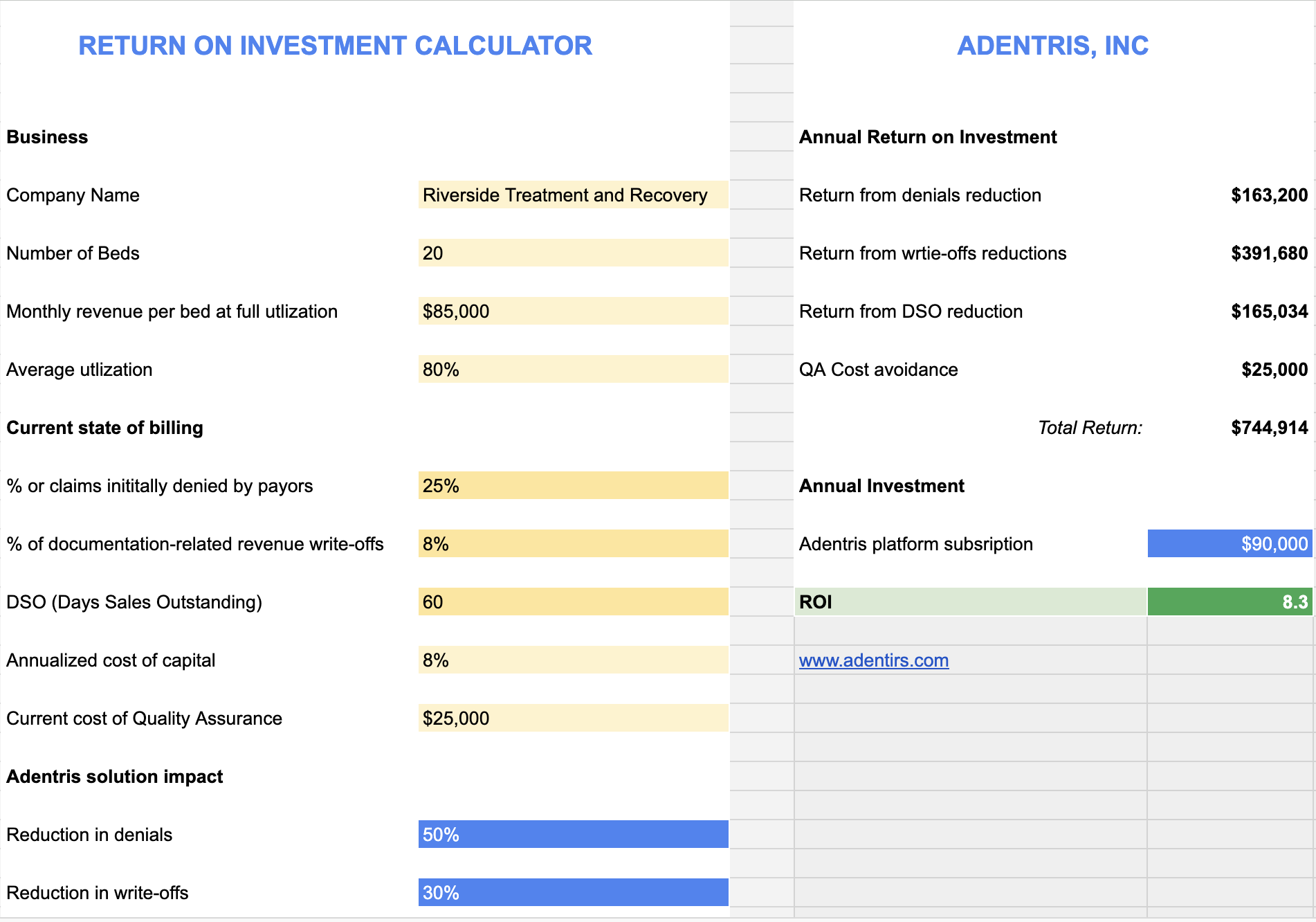
Return on Investment
The case is built on conservative gains, to show what a mid-sized clinic (say 1,000 anticoagulated patients) might see over a 12-month period:
- Baseline TTR: 55 %; after intervention: +8 pp → ~63 %
- Unaddressed out-of-range INR events (>48 hrs delay): 30 % → 15 % (50% reduction)
- Missed INR draws: 15 % → 8 % (≈ 45% reduction)
- Major bleeding rate: 3.5 /100 patient-years → 2.7 /100 (≈ 23% reduction)
- Stroke / thromboembolic events: 2.0 /100 → 1.6 /100 (20% reduction)
Financially, assuming:
- Cost per major bleeding hospitalization: $30,000
- Cost per stroke / thromboembolic hospitalization: $25,000
- Lab + clinic follow-up + staff monitoring cost per patient per year: ~$400
Then:
- If baseline bleed events are 35/year (3.5/100) → reduction to 27/year = 8 fewer events → 8 × $30,000 = $240,000 saved
- If stroke events are 20/year → reduced to 16/year = 4 fewer = $100,000 saved
- Avoiding monitoring inefficiencies and reducing labs/staff time wasted may save e.g. 200 patients × savings in staff / lab costs = assume $50/patient = $10,000
- Total savings ~ $350,000–$400,000 in direct care costs, plus indirect benefits (reduced complications, improved reputation, fewer readmissions, better payor relationships).
Sample Pilot Proposal: What We Can Do Together
Adentris would like to partner with hospitals or clinics for a pilot of our AI-powered real-time EMR/EHR quality intelligence platform customized on anticoagulation management. Key elements:
- Scope & Duration
- Duration: 6 months
- Patient population: warfarin + DOAC patients (you define cohort: e.g. AFib, VTE etc.)
- Data sources: EMR/EHR, lab systems, med-admin / pharmacy, patient scheduling if relevant
- What We Will Deliver
- Automated alerts for: out-of-range INR not addressed within preset thresholds; high INR variability; likely drug-drug interactions; patients with missed labs; possibly excess bleeding risk flags (renal insufficiency, age, interacting medications)
- Data integrity checks (missing labs, wrong dose entries, missing renal function etc.)
- Real-time dashboards of the key quality measures listed above (TTR, out-of-range labs, delays in patient contact, guideline compliance etc.)
- Monthly progress reports comparing baseline to current performance, and suggestions for targeted interventions (e.g. adjusting monitoring interval, provider workflows, patient education)
- Expectations & Measures of Success
- Baseline measurement period (first 1-2 months) to establish current performance on key metrics.
- Mid-pilot and end-pilot measurements on:
• TTR improvement
• Reduction in unaddressed out-of-range INR events and contact delays
• Reduction in missed lab draws
• Reduction in inappropriate DOAC dosing or drug interactions
• Reduction in major adverse events (if sufficient sample size)
- What We'll Need from You
- Access to de-identified (or appropriately consented) EMR/EHR and lab data, med prescribing data, patient demographic / risk factor data (age, renal function, comorbidities). Pilot can be effectively conducted without EMR/EHR integration.
- Some clinician / staff time for reviewing alerts, modifying workflows, addressing flagged issues
- What You Get
- All alerts, reports etc.
- Insights into where providers are risking safety, efficiency, cost, compliance
- Shared benchmarking vs peer institutions (anonymized)
Why Now, Why Partner With Adentris
- Many hospitals are under pressure both from payors and regulators to reduce adverse events, reduce readmissions, and demonstrate high-quality care. Anticoagulation is a known high-risk, high value area.
- Real-time feedback loops are increasingly recognized as essential: not waiting for quarterly reporting.
- The cost of failure (bleeds, strokes, litigation, reputational damage, patient harm) is high; the opportunity cost of not doing QI rigorously is also high.
Adentris brings:
- Proven AI‐assisted data pipelines that connect to EHR/EMR, lab, prescription, and pharmacy data.
- Existing algorithms for detecting out-of-range labs, dosing errors, drug interactions, missed labs, and risk stratification.
- Dashboard, alert engine, reporting system.
- Experience with clinical users to ensure alerts are actionable, not overwhelming.
Quality in anticoagulation management is measurable, improvable, and financially rewarding. By embedding AI-powered real-time QA into EMR workflows, hospitals and clinics can reduce harm, improve outcomes, and recover millions in avoidable costs.
Adentris is ready to partner with forward-looking institutions to make this transformation real. If your hospital or clinic is interested in joining the pilot program, contact us at sales@adentris.com